Case Study Series
Advanced wound care of perivulvar-pelvic necrotizing fasciitis using MiroDerm
By Brian Parkes, MD, FACS
Medical Director of Scotland Wound Healing, Scotland Memorial Hospital, Laurinburg, North Carolina
Overview
- 66-year-old female
- Poorly controlled diabetes
- BMI 42
Presenting Symptoms
- Presented to ED with 5-day history of pelvic pain, demonstrating signs and symptoms of sepsis
- Temp 103°, BP 98/56, pulse 110
- WBC 14.6, Hgb. 11.8, blood sugar 257
- Cultures: Bacteroides vulgaris
Physical Findings
Right perivulvar area revealed a small draining sinus, marked tenderness, and no crepitus.
Evaluation
CT scan showed a large gas collection measuring 13L x 8W x 6D cm in the soft tissues of the medial right thigh near the vulva and rectum to the inferior margin of the symphysis pubis to the level of the proximal thigh. The findings were consistent with a gas-forming organism.
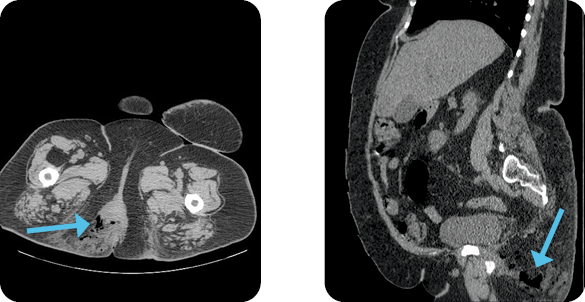
Diagnosis
Perivulvar-pelvic necrotizing fasciitis
Treatment
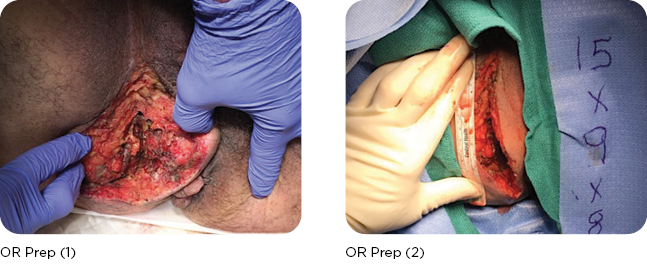
A large necrotizing infection in the perivulvar/perirectal space was debrided to the coccyx. The post-op wound was 15L x 8W x 9D cm. Wound V.A.C. was applied. The wound was re-debrided and irrigated, and negative pressure suction device (NPSD) was applied several times over the next three weeks, prior to the first MiroDerm Fenestrated application.
MIRODERM FENESTRATED
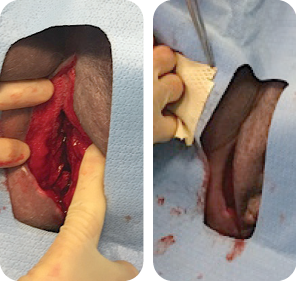
MIRODERM FENESTRATED DAY 1
Wound size: 10L x 6W x 8.5D cm
The wound was irrigated, and MiroDerm Fenestrated (8 x 15 cm) was applied. NPSD followed.
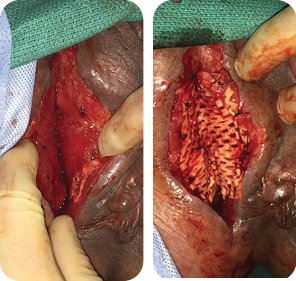
MIRODERM FENESTRATED DAY 7
Wound size: 10L x 3W x 4D cm
A second application of MiroDerm Fenestrated (3 x 7 cm) was applied by placing 3-0 Vicryl® sutures in the center of the mesh and then parachuting down into the apex of the wound. Additional fixation points of 3-0 Vicryl® were placed every 1-2 cm across the biologic to allow openings of 2-3 mm of the interstices.
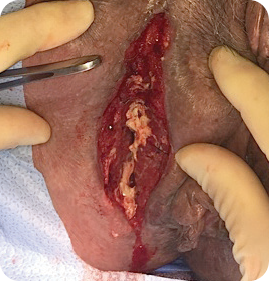
MIRODERM FENESTRATED DAY 12
Wound size: 10L x 1.8W x 2.5D cm
MiroDerm Fenestrated was still present in the wound and integrating with newly forming granulation. Loose product was re-approximated to the defect in lieu of new product. (No MiroDerm Fenestrated reapplication at this time.) Patient was discharged home with greater than 90% reduction in wound volume in less than two weeks.
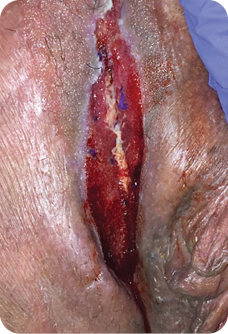
MIRODERM FENESTRATED DAY 21
Wound size: 8L x 1W x 1D cm
Most of the wound was less than 1 mm and was closed.
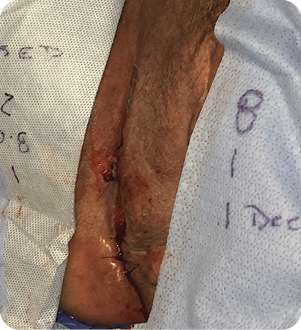
MIRODERM FENESTRATED DAY 21
Wound was sutured to close in 21 days after two MiroDerm Fenestrated applications. The remaining wound was 2L x 0.8W x 1D cm. No MiroDerm Fenestrated was applied, and NPSD was removed.
Discussion
Soft tissue infections are diverse in nature, and most commonly involve the subcutaneous tissue, fascia or muscle. They occur most frequently in the perineum, lower extremities and abdominal wall, and can be life threatening at times, even when appropriately treated.
Etiology: 20% are idiopathic, while others derive from skin conditions such as contaminated surgical incisions, chronic dermatitis, animal or human bites, and contaminated needles.
Surgical care consists of resuscitation, physiologic support, broad spectrum antibiotics, and urgent and thorough debridement.
Mortality from such infections is 29%1,2, and approximately 25%2 require a colostomy.
Postoperative wound care has traditionally consisted of wound vacs and skin grafting. More novel approaches with biologics such as MiroDerm Fenestrated are currently in use.
Contact us for more info on MiroDerm
The experience depicted in this case study may not be representative of all potential clinical outcomes. MiroDerm Fenestrated results may vary by patient. Further study is necessary to determine the benefit of MiroDerm Fenestrated in a variety of wound applications.
1. Wong CH, et al. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003. 85a(8):1454-60.
2. McHenry, et al. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995. 221(5):558-565.
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2020 Reprise Biomedical. All Rights Reserved. SM-00041 Rev. D 07/20
