Case Study Series
Diabetic foot ulceration Successfully treated with MiroDerm Biologic Wound Matrix
By Raymond John Abdo, DPM Mercy Hospital South, St. Louis, MO
Dr. Raymond Abdo DPM is a foot and ankle surgeon in Saint Louis, MO specializing in Foot & Ankle Surgery and Podiatry (Foot & Ankle Medicine). Dr. Abdo is also in private practice at locations in St. Louis and Eureka, MO. He graduated from Des Moines University College of Podiatric Medicine and Surgery in 2002 and has 19 years of experience. Raymond Abdo DPM is affiliated with Mercy Hospital – St. Louis and Mercy (Arkansas, Kansas, Missouri, and Oklahoma).
Overview
- 67-year old male
- Diabetes
- Neuropathy
- Gout
- Hyperlipidemia
- CAD
- Previous aortic aneurysm
- Previous I&D of right foot
- Previous left mid-foot amputation
Presenting Symptoms
- Following yard work, patient complained of increased redness and warmth on right foot
- Fever, chills, loss of appetite
- T=98.9, R=18, BP=127/51, Pulse=80
- WBC=10.4, Hgb=11.9, plt=121, glucose=302
Physical Findings
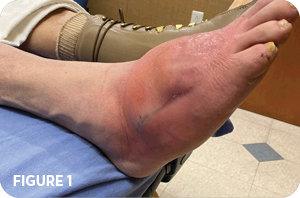
- Palpable pulses on right with positive edema noted (Figure 1)
- Increased redness, warmth, and swelling with fluctuance during palpation
- Rocker bottom deformity on right foot indicative of residual charcot deformity
Diagnosis
- Radiograph revealed abscess of right foot
- MRI positive for osteomyelitis
Treatment
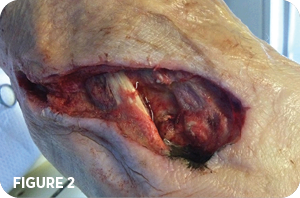
Incision and drainage of right foot abscess performed in O.R. The initial wound measured 3cm x 10cm x 1.3cm deep (Figure 2). No resection of bone was performed. Culture was negative for osteomyelitis. Silver alginate dressing with a dry dressing was applied afterwards. A wound vac was attempted to be applied the next day but excessive bleeding noted. Continued with same dressings over the next six days.
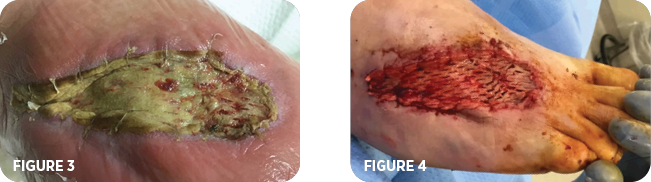
On the seventh day, the patient returned to the O.R. The wound bed was prepared and the MiroDerm wound matrix 7cm x 10cm was applied (Figure 3) in addition to primary and secondary dressings which included xeroform, silver alginate and a dry dressing. The next day a wound vac was applied at the bedside, IV antibiotics were prescribed, and the patient was discharged. A week later the patient was readmitted to the hospital with complaints of increased redness, warmth and swelling of the right foot. Lab values indicated no infection. The patient demonstrated non-compliance by ambulating on his right foot. The increased weightbearing may have contributed to increased inflammation of the right foot. Elevation and rest provided a decrease in redness, warmth, and swelling. Patient was discharged on the same day with a wound vac and IV antibiotics. Four weeks later the patient was seen in the clinic for a follow-up visit. The wound, now measuring 9.5cm x 2cm x 0.2cm (Figure 4), was debrided and a second application of MiroDerm was applied.
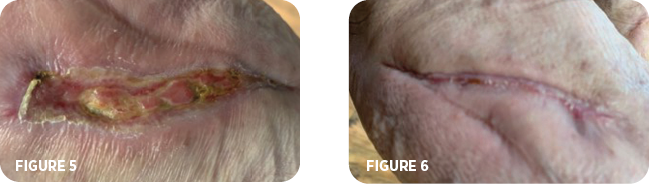
The wound vac was discontinued. The patient was prescribed gentamycin cream to apply to the ulceration site. IV antibiotics continued. Following an additional four weeks the patient’s wound measured 4.5cm x 0.7cm x 0.1cm (Figure 5). Antibiotics were discontinued. Local application of a gentamycin cream was still being applied. MiroDerm applications were no longer deemed necessary. The patient continued with follow-up appointments over the next month. By the next four weeks, approximately 24 weeks from when the patient initially was diagnosed with an abscess of the right foot, the wound was completely healed (Figure 6).
Summary
This is a clear demonstration of the adaptability and flexibility of MiroDerm wound matrix. In this case study, the hepatic derived collagen matrix was placed over exposed tendon representing deep structure. The wound covering can also be used in shallower wounds. MiroDerm provided an environment that was conducive to improved cellular proliferation, collagen activity, and fibroblastic activity that led to a rapid, successful wound closure.
Contact us for more info on MiroDerm
The experience depicted in this case study may not be representative of all potential clinical outcomes. MiroDerm Fenestrated, results may vary by patient. Further study is necessary to determine the benefit of MiroDerm Fenestrated in a variety of wound applications. MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence). CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use. Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2022 Reprise Biomedical. All Rights Reserved. SM-00220 Rev. A 01/22
