
Three-dimensional solution for deep and tunneling wounds
New HCPCS Level II Code A2025

Miro3D is designed for use in deep and tunneling wounds
- 2cm thick collagen sheet scaffold designed for deep, tunneling and irregular wounds
- Open and porous acellular graft provides protective environment for wound management
- Cut to desired size for full wall apposition and conformity to wound beds
Unlike thin grafts, Miro3D provides volume in deep wounds

Broad range of clinical uses
- Stage III and IV pressure ulcers
- Necrotizing fasciitis
- Partial and full-thickness wounds
- Wound dehiscence
- Perirectal abscess
- Pilonidal sinus disease
- Diabetic foot ulcers
- Trauma wounds
- Other tunneled and undermined wounds
Presented at the Symposium on Advanced Wound Care (SAWC) Spring Innovation Theater, Friday, May 2, 2025
Speakers: John P. Kirby, MD, MS, CWSP, FACS; Raymond John Abdo, DPM; Walaya Methodius-Rayford, MD, MBA, RPVI, CWSP, DAVBLM, FACCWS, FAPWCA; Ryan Dirks, MS, PA, Founder and CEO United Wound Healing; Lucian G. Vlad, MD, Clinical Associate Professor, Director, Wound Care and Hyperbaric Fellowship, Atrium Health Wake Forest Baptist Wound Care and Hyperbaric Medicine Center, Plastic and Reconstructive Surgery Department, Wake Forest School of Medicine, Winston-Salem, NC.
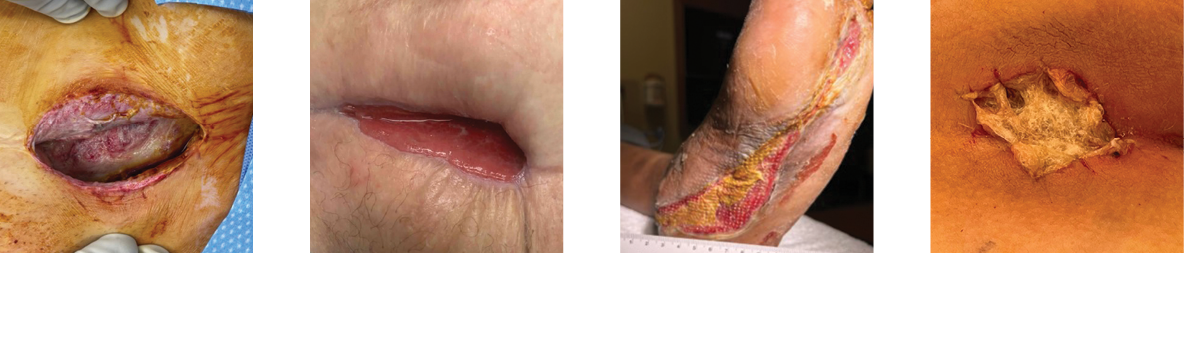
Clinical Case Studies and Posters*
Amputation Wound
Necrotizing Fasciitis
SAWC Fall 2023 Poster
Abdominal Surgical Dehiscence
Sebaceous Cyst
SAWC Spring 2024 Poster
Radiated Tissue Wound
Foot Abscess
SAWC Spring 2024 Poster
Foot Abscess
Pilonidal Sinus Abscess
SAWC Fall 2024 Poster
Available in six sizes that can be cut and shaped to fill and maintain direct contact with irregular wound beds

All sizes = 2cm thickness
Ordering Information
Available in a fiber version designed for irregular wound beds

Ordering Information
Product Inquiries and Orders
Call us at: 763-284-6780 Fax us at: 952-856-5085 Product Inquiries at: customerservice@reprisebio.com
SM-00198 Rev. P 06/25


