Our Products
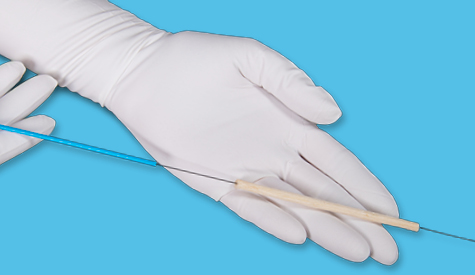
Designed for deep and tunneling wounds
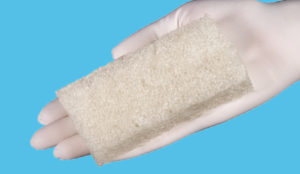
Designed to conform to any wound bed
Designed for delivery to tunneling wounds
Perfusion Decellularization Technology
With Reprise’s proprietary perfusion decellularization, the main vasculature of the liver is cannulated and perfused with a mild solution through the native vessels. The result is a preserved collagen scaffold with vasculature that retains strength and a micro-environment for cellular integration.
Events
SAWC Spring 2026 – April 9-11
Visit us at booth 1335 at the Symposium on Advanced Wound Care (SAWC), April 9-11 in Charlotte, North Carolina.
Conference Website >
News
Miro3D wound matrix adds two NEW sizes to its portfolio!
Customer Resources
Our Company
SM-00140 Rev. G 05/25