Case Study Series
Patient with retrorectal necrotizing fasciitis forgoes colostomy in favor of treatment with MiroDerm
Medical Director of Scotland Wound Healing, Scotland Memorial Hospital, Laurinburg, North Carolina
Overview
- 39-year-old male
- Previously undiagnosed diabetic
- BMI 38
Presenting Symptoms
- Presented to ED in shock
- Hypotensive and tachycardic
- Fever 104°, WBC 26,000, Glucose 278
- HgbA1-c 14
- Culture: Heavy beta strep
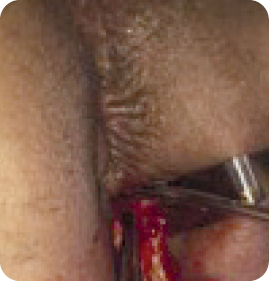
Physical Findings
Evaluation
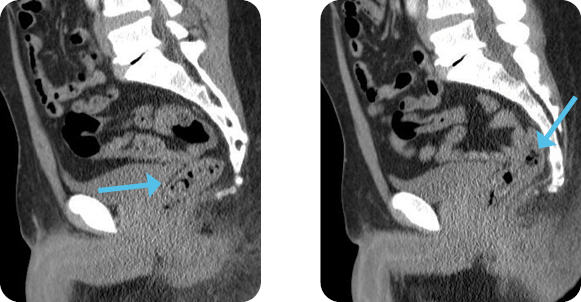
Diagnosis
Treatment
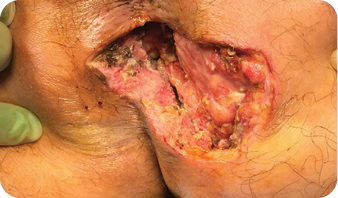
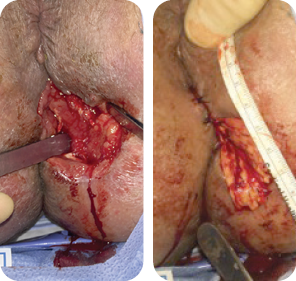
The wound underwent sharp debridement, cleansing with a Pulsavac® device, and
application of a Negative Pressure Suction Device (NPSD) for seven days.
MIRODERM FENESTRATED
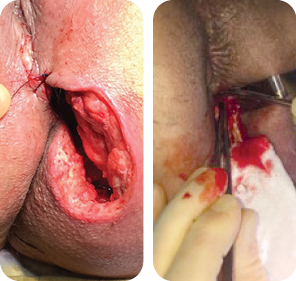
Wound size: 8L x 10W x 6.5D cm
Initial MiroDerm Fenestrated application (8 x 15 cm) occurred seven days post initial debridement. An anoplasty was performed, and VersaFoam® was placed over the MiroDerm Fenestrated.
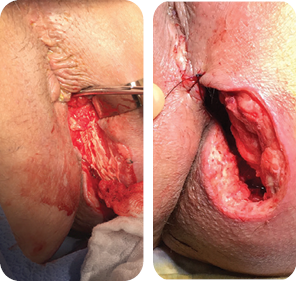
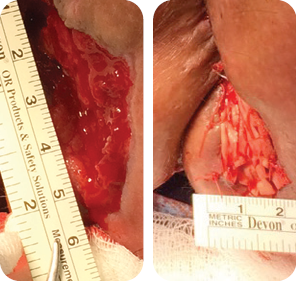
Wound size: 8L x 6W x 5D cm
Initial MiroDerm Fenestrated product had incorporated into wound, with a marked reduction in wound depth. MiroDerm Fenestrated (5 x 5 cm) was applied for the second time, and anoplasty was
repeated to facilitate Negative Pressure Wound Therapy (NPWT).
Wound size: 7.5L x 6W x 4D cm
The wound volume reduced by 65% since Day 1. MiroDerm Fenestrated was still present in the wound, and the remaining product was re-sutured. No additional MiroDerm Fenestrated was placed at this time.
Wound size: 5L x 3W x 2D cm
The wound volume reduced by more than 90% since Day 1. The remaining MiroDerm Fenestrated had integrated into granulation tissue. MiroDerm Fenestrated (3 x 7 cm) was placed for a third time.
Wound size: 5L x 2W x 2D cm
Three days after the third MiroDerm Fenestrated application, a nurse accidentally removed the NPSD and MiroDerm Fenestrated, necessitating a return to the OR on Day 27 for a fourth MiroDerm Fenestrated application (3 x 7 cm).
Wound size: 5L x 2W x 1D cm
NPWT was discontinued.
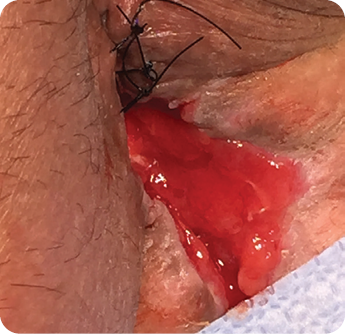
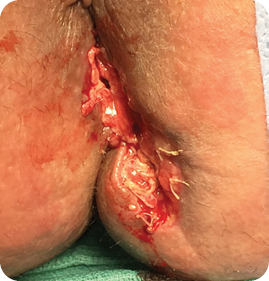
Wound size: 3.5L x 1W x 1D cm
Post-closure wound size: 2L x 1W x .5D cm
Patient was discharged.
The patient was discharged 35 days after the first MiroDerm Fenestrated application, with intact anoplasty and good sphincter control. The remaining wound was dressed with hydrogel and bio-occlusive dressing.
Discussion
Anoplasty and creative dressings with skin glue and DuoDERM® were required to maintain a wound
NPSD seal around the anus. Low intensity, continuous NPWT was performed with suction at 75 mmHg after placing the MiroDerm Fenestrated. Suction was reduced to control exudate rather than promote granulation tissue.
The patient healed in a remarkable amount of time and likely avoided at least two major debilitating surgeries, as well as the emotional and physical impact of a colostomy for 3-6 months. The
accelerated healing observed with MiroDerm Fenestrated is likely the result of Reprise
Biomedical’s unique perfusion decellularization technology, which retains a 3-dimensional
extracellular matrix that includes the vasculature.
Contact us for more info on MiroDerm
1. Wong CH, et al. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003. 85a(8):1454-60.
2. McHenry, et al. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995. 221(5):558-565.
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2020 Reprise Biomedical. All Rights Reserved. SM-00042 Rev. D 07/20
