Case Study Series
Necrotizing wound infection of the thigh
By Brian Parkes, MD, FACS, Medical Director of Scotland Wound Healing, Scotland Memorial Hospital, Laurinburg, North Carolina
Overview
- 67-year-old male
- Diabetes
- End-stage renal disease (ESRD) on dialysis
Presenting Symptoms
- Presented with septic shock
Diagnosis
- Necrotizing wound infection of the left anterior thigh
Treatment
- The patient was taken to the OR where all skin, fat, and fascia was removed from the inguinal crease to near the knee on the left leg
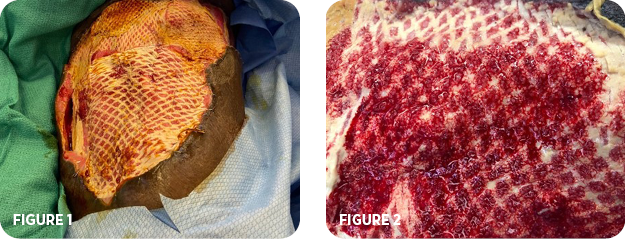
- Two repeat trips to the OR were required to remove all necrotic tissue before MiroDerm was applied (Figure 1)
- A VAC was applied on top of the MiroDerm at 75mmHg and left in place for 1 week
- Infiltration was observed at the 1-week dressing change (Figure 2)
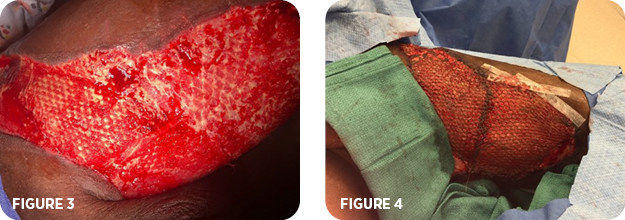
- By week 2, the wound was healed to skin level with a healthy bed of granulation tissue (Figure 3)
- A split-thickness skin graft (STSG) was applied (Figure 4)
- The patient was discharged a week later with 90% take on his skin graft
Summary
After all necrotic tissue was removed from the wound bed, MiroDerm was placed and was able to heal the wound to skin level in just 2 weeks, at which point the patient was ready for the final stage of the healing process, a split thickness skin graft, to which he responded incredibly well.
Discussion
In clinical practice, MiroDerm can be utilized until granulation tissue has reached skin level and the wound is ready for a split-thickness skin graft.
Placing a pristine, hepatic-derived collagen matrix, MiroDerm, into a wound provides an environment that is conducive to improved proliferation of extracellular matrix (ECM), collagen, and fibroblastic activity, which leads to rapid and successful wound closure.
Placing a pristine, hepatic-derived collagen matrix, MiroDerm, into a wound provides an environment that is conducive to improved proliferation of extracellular matrix (ECM), collagen, and fibroblastic activity, which leads to rapid and successful wound closure.
Contact us for more info on MiroDerm
The experience depicted in this case study may not be representative of all potential clinical outcomes. MiroDerm Fenestrated, results may vary by patient. Further study is necessary to determine the benefit of MiroDerm Fenestrated in a variety of wound applications.
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2021 Reprise Biomedical. All Rights Reserved. SM-00194 Rev. A 02/21
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2021 Reprise Biomedical. All Rights Reserved. SM-00194 Rev. A 02/21
