Case Study Series
Medial thigh flap necrosis treated with MiroDerm Biologic Wound Matrix
By Moses K. Shieh, DO, FACOS, General, Bariatric & Cosmetic Surgery, Surgical Healing Arts Center, Fort Myers, Florida
Medical School at Des Moines University, Iowa. General Surgery, Mount Clemens Regional Medical Center. Fellowship in Bariatric & Cosmetic Surgery.
Medical School at Des Moines University, Iowa. General Surgery, Mount Clemens Regional Medical Center. Fellowship in Bariatric & Cosmetic Surgery.
Overview
- 50-year-old female, history of gastric bypass with an initial BMI 50 and current BMI 29.
- Five days before being seen in the clinic, she had a bilateral medial thigh lift to remove the hidradenitis and excess skin after the massive weight loss.
- The patient has chronic iron deficiency anemia due insufficient iron absorption.
Presenting Symptoms
- Patient’s vital signs were normal.
- No other remarkable findings during the initial consult.
Diagnosis
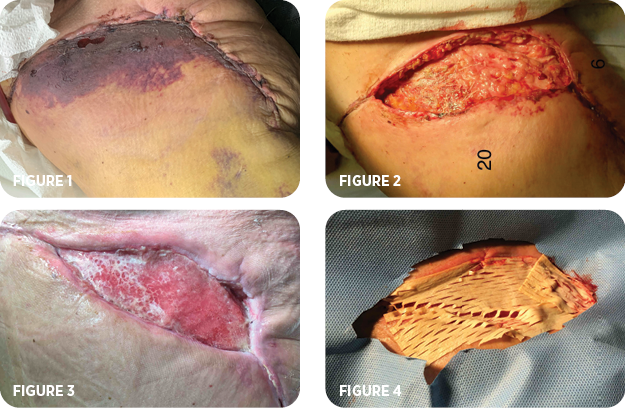
- Full-thickness necrosis of 19cm x 5cm of the right thigh dermal flap.
Treatment
- The necrotic tissue was excised (Figure 1), and negative pressure wound therapy (NPWT) was applied for 14 days (Figure 2).
- NPWT was discontinued after the wound bed was fully granulated. A sheet of MiroDerm, perfusion decellularized porcine hepatic-derived wound matrix, was placed (Figures 3 & 4).
- The matrix was secured with five sutures of 3-0 poliglecaprone, a silicone adhesive dressing, and an ABD pad. Daily ABD pad changes were performed, as well as irrigation of the wound over the silicone dressing with saline solution. The patient was followed closely at the office.
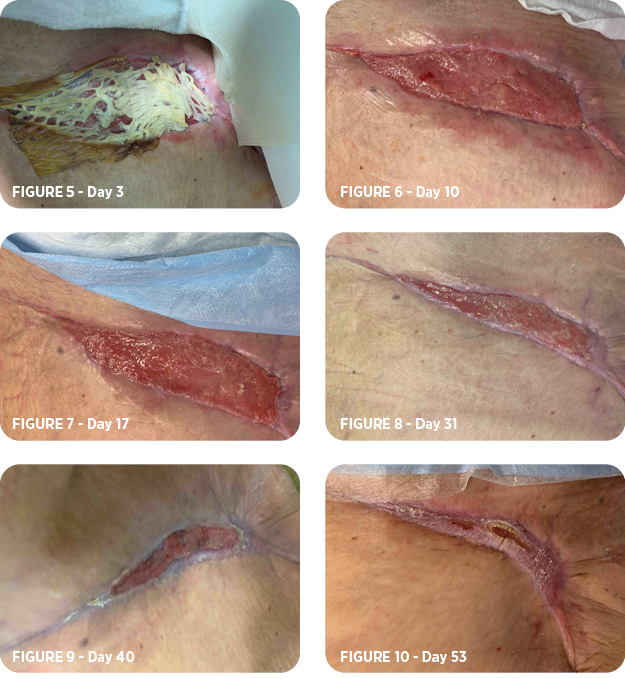
- After the initial application of MiroDerm, follow-up occurred on day 3 (Figure 5), day 10 (Figure 6), day 17 (Figure 7), day 31 (Figure 8), day 40 (Figure 9), and day 53 (Figure 10).
- MiroDerm was fully integrated 10 days after placement. The recommendations for the patient were daily dressing changes after showering and return to work. The wound was completely healed, and follow-up concluded 8 weeks after the initial application of MiroDerm.
Summary
Necrosis of the skin is a rare complication of a medial thigh lift; it leaves a large wound defect without the option of other immediate flap rotation. MiroDerm is a hepatic-derived collagen matrix that provides exceptional rapid healing of a significant dermal defect, avoiding returning to the OR, and keeping the treatment as an outpatient.
Contact us for more info on MiroDerm
The experience depicted in this case study may not be representative of all potential clinical outcomes. MiroDerm Fenestrated, results may vary by patient. Further study is necessary to determine the benefit of MiroDerm Fenestrated in a variety of wound applications.
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2021 Reprise Biomedical. All Rights Reserved. SM-00204 Rev. A 06/21
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2021 Reprise Biomedical. All Rights Reserved. SM-00204 Rev. A 06/21
