Case Study Series
Foot ulceration with bone resection successfully treated with MiroDerm
By Raymond John Abdo, DPM Mercy Hospital South, St. Louis, MO
Dr. Raymond Abdo DPM is a foot and ankle surgeon in Saint Louis, MO specializing in Foot & Ankle Surgery and Podiatry (Foot & Ankle Medicine). Dr. Abdo is also in private practice at locations in St. Louis and Eureka, MO. He graduated from Des Moines University College of Podiatric Medicine and Surgery in 2002 and has 19 years of experience. Raymond Abdo DPM is affiliated with Mercy Hospital – St. Louis and Mercy (Arkansas, Kansas, Missouri, and Oklahoma).
Overview
- 43-year old female
- Obesity
- Neuropathy
- Hypertension
- Extensive past surgical history
- Permanent hardware in foot and ankle (non-lateral side)
Presenting Symptoms
- Increasing redness, warmth, and swelling on left foot
- Pulse noted with hair growth
- Radiograph negative
- Bone scan positive for uptake in lateral left foot, representing osteomyelitis
- Neurological protective threshold limited on left
- Rigid cavus structure on left foot with no crepitus at observed ulceration
- WBC=WNL; afebrile
Diagnosis
- Admitted to hospital for cellulitis and ulceration on lateral left 5th metatarsal
Treatment
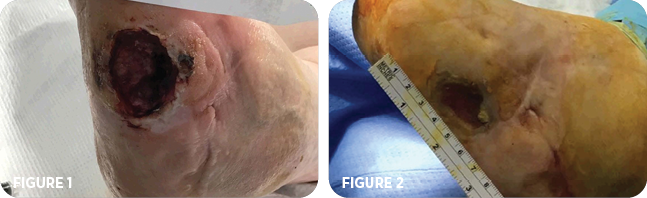
Upon consultation, patient refused amputation of left 5th metatarsal. Therefore, patient was brought to the O.R. for excisional debridement of ulceration including bone on left foot. Post debridement, ulceration measured 4cm x 3cm x 1.5cm deep. (Figure 1).
One day post surgery, a wound vac was applied at bedside. Cultures were positive for MRSE. The patient was placed on IV antibiotics and was discharged from the hospital with the wound vac and home health follow-up.
Six weeks later, the patient returned to the clinic for a follow-up visit. The ulceration measured 2.5cm x 3cm x 1cm deep (Figure 2) with positive bone probe with red, clean granular base noted. Patient continued with wound vac therapy and IV antibiotics and was scheduled for excisional debridement of ulceration including bone with fluoroscope guided needle placement.
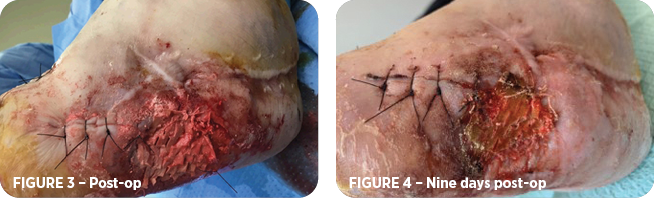
Five days later, patient returned to the O.R. for resection of proximal left 5th metatarsal and application of Bonesync™ (bone filler) and vancomycin to fill the bone resection void. MiroDerm wound matrix was applied at the ulceration site (Figure 3) and covered with xeroform alginate bandage and a secondary dry dressing. Prior to the procedure, the ulceration measured 2.5cm x 3cm x 0.2cm deep and was slightly larger following the resection.
Nine days post-op, ulceration measured 2cm wide x 0.1cm deep. The sutures on the ulceration and the wound covering remained intact and clean (Figure 4). The primary and secondary dressings were changed. No constitutional symptoms were reported. Patient was removed from wound vac and antibiotics discontinued. The patient was asked to remain non-weight bearing and return for follow-up in one week.
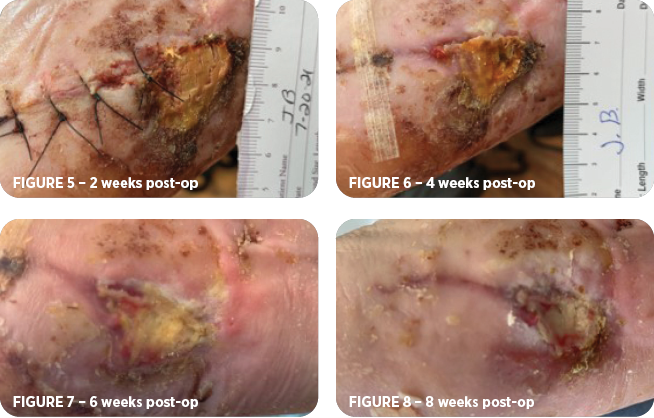
Approximately one week later, or two weeks post-op, there were no signs of infection and no notable changes in wound size (Figure 5). The sutures were removed. The MiroDerm remained intact and outer dressings were changed. The patient remained non-weight bearing.
One week later, or three weeks post-op, it was noted the bone filler had settled following the removal of the stitches the week prior and the wound measurements were increased to 3cm x 2cm x 0.2cm deep; however, red granulation tissue was present under MiroDerm with minimal fibrous tissue. Active debridement performed without disrupting integrated portions of MiroDerm.
Over the next five weeks, the patient was seen at follow-up visits every one to two weeks and underwent active debridement and primary and secondary dressing changes. The wound continued to reduce in size with continued observation of red granulation tissue and no signs of infection (Figures 6-8).
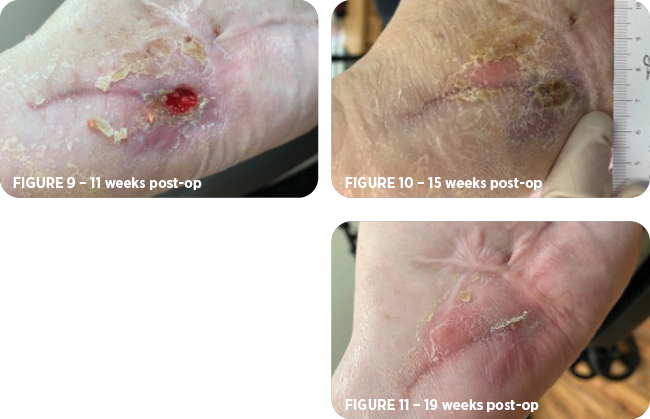
At 11 weeks post-op, the wound measured 1cm x 0.5cm x 0.2cm deep (Figure 9) and another MiroDerm was applied with a bolster dressing to secure it in place. The patient continued with NWB instructions.
Within two weeks, it was noted that MiroDerm had integrated well into the wound bed. No signs of infection. Debridement was not performed.
15 weeks post-op, the ulceration was considered fully healed (Figure 10) and the patient was to remain NWB until a custom brace was fitted.
The patient followed up after four weeks still with a fully healed wound (Figure 11). By this time, the patient had returned to weight bearing with a brace and had been attending physical therapy.
Summary
This case demonstrates positive clinical results of simultaneously using a bone filler and MiroDerm to fully heal a bone resection and surgically treated ulceration. MiroDerm was used in the early post operative phase which assisted in the creation of healthy granulation tissue and then again in the final healing stage to assist in fully closing the wound.
Contact us for more info on MiroDerm
The experience depicted in this case study may not be representative of all potential clinical outcomes. MiroDerm Fenestrated, results may vary by patient. Further study is necessary to determine the benefit of MiroDerm Fenestrated in a variety of wound applications.
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2022 Reprise Biomedical. All Rights Reserved. SM-00221 Rev. A 02/22
