
Perfusion decellularized hepatic surgical matrix primed for cellular integration

Perfusion decellularized hepatic surgical matrix primed for cellular integration
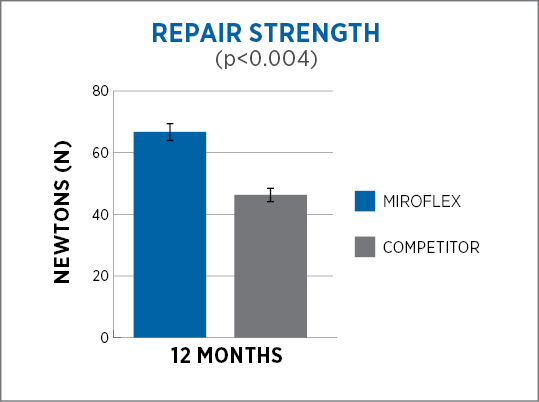
Designed for soft tissue reinforcement
Unique hepatic-derived surgical matrix that provides an open collagen scaffold with natural vascular pathways; MiroFlex is primed for cellular integration.
- For use in open and laparoscopic hiatal, paraesophageal, parastomal, and ventral hernia repairs
- Ability to be custom shaped and fit through a laparoscopic trocar
- Can be quilted together to reinforce larger hernias
MiroFlex provides a greater degree of cellular integration and remodeling1, and significantly greater strength of repair2 than a dermis-derived competitor* as shown in animal studies.
MiroFlex has natural vascular pathways and an open collagen matrix that facilitates the initiation of cellular ingrowth and tissue remodeling.
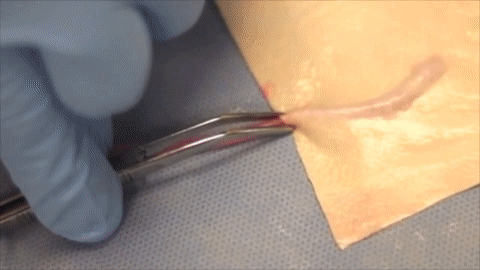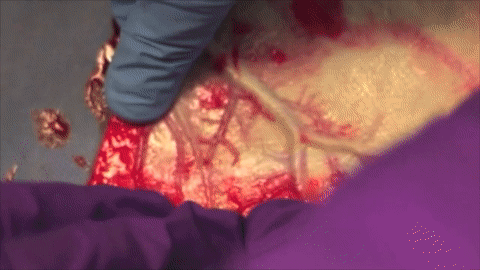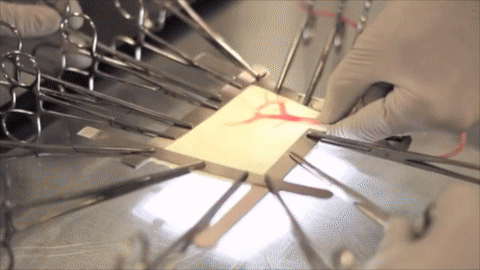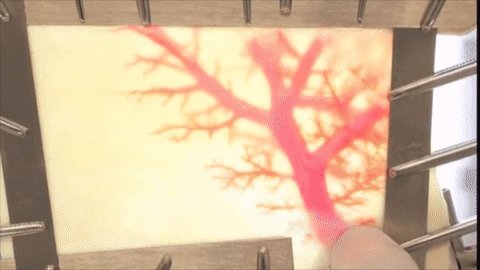
A vessel on the open edge of MiroFlex was cannulated and a red dye was injected into the graft to demonstrate the presence of the native liver vasculature, including the capillaries.
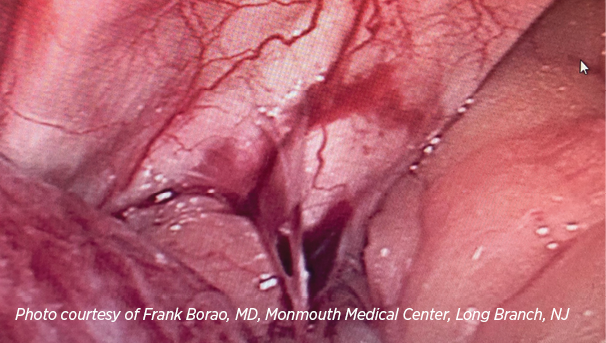
MiroFlex shows diaphragmatic incorporation 6 months after paraesophageal hernia repair, as noted during cholecystectomy.
Clinically proven for hiatal and paraesophageal hernia repair
Clinical trials evaluated the long-term results when MiroFlex was used to reinforce the primary crural closure during hiatal and paraesophageal hernia repair (PEHR).
Paraesophageal Hernia Trial4
Prospective, multi-center, 41 patients, two-year follow up*
Primary endpoint: Hernia recurrence requiring surgical reintervention at two years post operatively due to symptoms or adverse events.
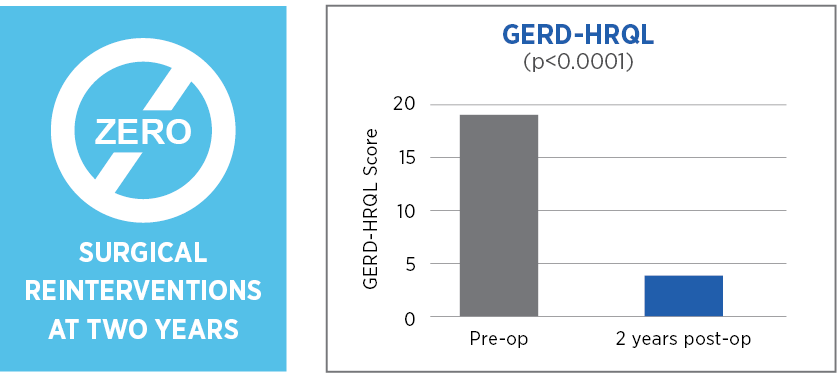
MiroFlex is safe and effective for crural reinforcement in laparoscopic PEHR and did not result in any mesh-related complications.
- No major intraoperative complications reported.
One case of perioperative mortality in a patient that arrested on postoperative day 1. - 18 postoperative adverse events (4 serious); none device related.
*27 patients completed two-year follow up
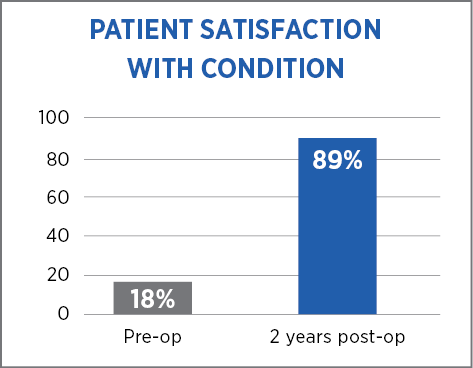
The high patient satisfaction rate at two years suggests that the addition of MiroFlex to the hiatal hernia repair is effective.
Hiatal Hernia Trial5
Retrospective review with prospective follow up, single center, 85 patients, 1.3 years (mean) follow up*
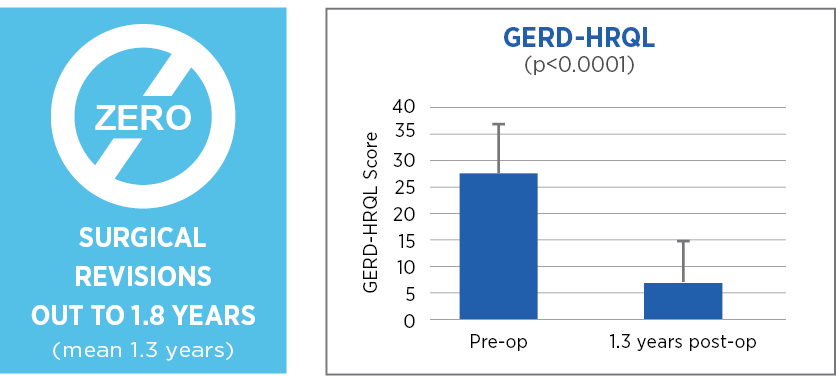
Laparoscopic Nissen fundoplication with MiroFlex is a safe procedure with no mesh-related complications over a short to medium follow up.
Three perioperative complications resolved without treatment and were not device related.
*73 patients followed out to 1.8 years (mean 1.3 years)
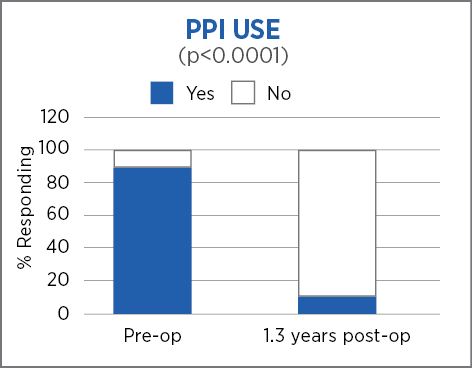
Excellent outcomes and no revisions a mean of 1.3 years after surgery suggest that a durable repair had been achieved.
Clinical Data
| STUDY | AUTHOR | PUBLICATION | FULL ARTICLE |
| An in vivo analysis of MiroMesh – a novel porcine liver prosthetic created by perfusion decellularization | Clayton C. Petro, MD | Journal of Surgical Research 2016;201:29-37 |  Read Here Read Here |
| A comparative analysis of ventral hernia repair with a porcine hepatic-derived matrix and porcine dermal matrix | Job Tharappel | International Journal of Abdominal Wall and Hernia Surgery 2019;2(3):89-95 |  Read Here Read Here |
| A multi-center, prospective clinical trial of a hepatic derived porcine surgical mesh for the laparoscopic repair of symptomatic paraesophageal hernias | Michael J. Rosen | The American Journal of Surgery 2019;218(2):315-322 |  Read Here Read Here |
| Retrospective review and prospective follow-up of 85 consecutive patients treated with a novel hepatic-derived surgical mesh for hiatal hernia repair: outcomes, surgical complications, and revisions | George K. Gillian, MD | Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2019;29(6):529-533 |  Read Here Read Here |
Education
| TITLE | PHYSICIAN | ASSOCIATION | DATE | VIDEO |
| Reducing Failure Rates in Hiatal and Paraesophageal Hernia Repair – the ‘Achilles Heel’ of Anti-reflux Surgery | Dr. G. Kevin Gillian | Florida Chapter, American College of Surgeons |
01/19/2021 |  Watch Here |
Ordering Information
| ITEM ID | SIZE | TOTAL cm2 |
| BLM-100-01-0608 | 6 x 8 cm | 48 |
| BLM-100-01-0808 | 8 x 8 cm | 64 |
| BLM-100-01-1010 | 10 x 10 cm | 100 |
| BLM-100-01-0816 | 8 x 16 cm | 128 |
| BLM-100-01-1016 | 10 x 16 cm | 160 |
| BLM-100-01-1020 | 10 x 20 cm | 200 |
MiroFlex is processed and stored in a phosphate buffered aqueous solution, packaged in an inner sterile pouch and outer non-sterile pouch, and sterilized with electron beam irradiation. MiroFlex comes in a variety of sizes and is intended for use only by trained medical professionals who are familiar with surgical procedures and techniques involving surgical mesh. Federal (U.S.A) law restricts this product to sale by or on the order of a physician.
MiroFlex Biologic Matrix is intended to be implanted to reinforce soft tissue and is also intended for implantation to reinforce soft tissue where weakness exists in patients requiring soft tissue repair or reinforcement in plastic and reconstructive surgery.
See IFU for full prescribing information, including indications, contraindications, precautions, and potential complications.
References:
- Petro, CC; Prabhu, AS; Liu, L; Majumder, A; Anderson JM; Rosen, MJ. An in vivo analysis of MiroMesh – a novel porcine liver prosthetic created by perfusion decellularization.
Journal of Surgical Research. 2016. 201:29-37. - Tharappel, J; Wennergren, JE; Lee, EY; Madabhushi, VV; Plymale, MA; Roth, JS.A comparative analysis of ventral hernia repair with a porcine hepatic-derived matrix and porcine
dermal matrix. International Journal of Abdominal Wall and Hernia Surgery. 2019. - Reprise Biomedical data on file.
- Rosen, MJ; Borao, FJ; Binenbaum, SJ; Roth, JS; Gillian, GK; Gould, J; Heniford, BT. A multi-center, prospective clinical trial of a hepatic derived porcine surgical mesh for the
laparoscopic repair of symptomatic paraesophageal hernias. The American Journal of Surgery. 2019. 218(2):315-322. - Gillian, KJ; Bansal, D. Retrospective review and prospective follow-up of 85 consecutive patients treated with a novel hepatic-derived surgical mesh for hiatal hernia repair.
Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2019.
*Strattice™ Reconstructive Tissue Matrix (Allergan, Dublin, Ireland)
Product Inquiries and Orders
Call us at: 763-284-6780
Fax us at: 952-856-5085
Product inquiries at: customerservice@reprisebio.com
SM-00154 Rev. F 08/22

