Prospective Multi-Center Study
MiroDerm hard-to-heal DFUs clinical study¹
Study investigators: Robert Fridman, DPM, Columbia University Medical Center, New York, NY • Payam Rafat, DPM, A Step Ahead Foot Care, Frisco, TX • Carl Van Gils, DPM, Foot and Ankle Institute, St. George, UT • Deena Horn, DPM, Weill Cornell Medicine, New York, NY • Dean Vayser, DPM, ILD Research Center, Carlsbad, CA • C. Jake Lambert, MD, Clinical Research of Central Florida, Winter Haven, FL
Study Overview
The purpose of this single-arm, multi-center (9 sites), prospective study was to evaluate the ability of a novel Hepatic-Derived Wound Matrix (HD-WM), our MiroDerm, to treat difficult-to-heal
diabetic foot ulcers that had been present for at least 90 days and were non-responsive to at least two applications of an advanced biologic wound care product. 53 patients were enrolled in this study and 38 completed per protocol. The study subjects had wounds that had not healed within the previous 3 months. Subjects were fitted for, instructed on the use of, and received either a DH
off-loading walker of a DH off-loading post-op shoe with a customizable peg insert.
Subject Demographics
- 73.7% Male
- Mean Age: 61.7
- Mean BMI: 31.2
- Mean HbA1c: 7.8
- Mean ABI: 1.02
Primary Outcome
Proportion of healed DFUs at 12 weeks
Complete wound closure defined as: 100% epithelialization, no exudate, no need
for further dressing
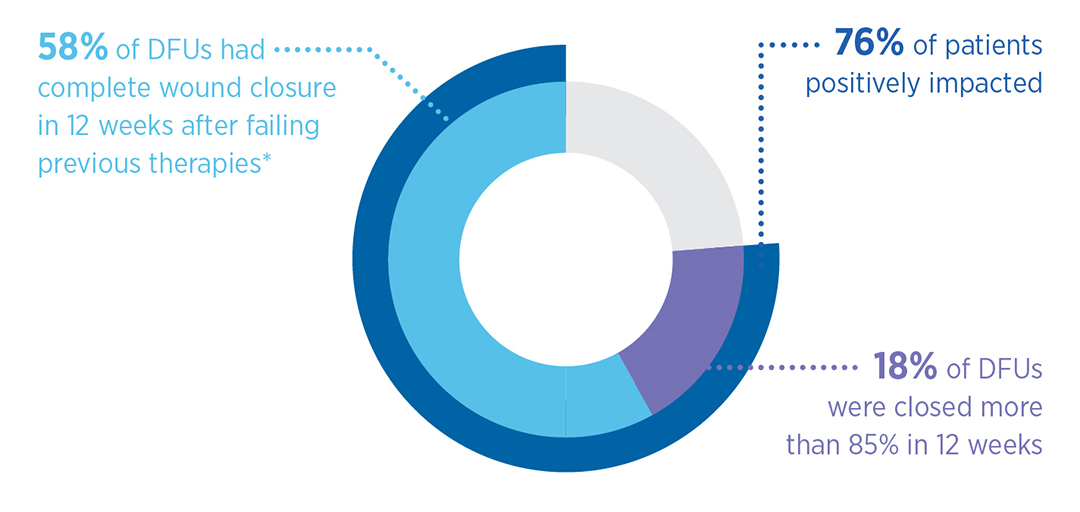
Secondary Outcome
- Mean time to closure of healed cohort: 8.1 weeks
- Mean number of MiroDerm applications in healed cohort: 2.1
- Percent closed over time: 71.4%
Conclusion
In this study, MiroDerm closed DFUs that had previously failed other advanced therapies. These favorable results are consistent with a similar pilot study.2 The results indicate that this HD-WM shows a robust potential to manage wounds and is an
appropriate technology to treat difficult-to-heal DFUs.
Failed advanced biologic applications
Prior to being treated with MiroDerm, subjects received a mean of 3.2 other advanced biologic applications.
Types of biologics previously used are quantified in the table below.*
| Previous Advanced Therapy | Subjects Receiving at Least One Application |
Total Number of Applications |
| EpiFix® | 12 | 30 |
| REGRANEX® Gel | 7 | 8 |
| Cytal® | 5 | 9 |
| Epicord® | 5 | 5 |
| PriMatrix® | 3 | 4 |
| Kerecis™ Omega3 | 3 | 6 |
| Integra® Bilayer | 3 | 5 |
| Omnigraft™ | 3 | 5 |
| Other | 9 | 29 |
*Key Inclusion Criteria: failed at least 2 attempts with an alternative advanced biologic, DFU that has not healed in the previous 3 months, neuropathic DFU between 1 and 12 cm2, less than 5 mm deep, full thickness, below the ankle, and with no exposed bone or tendon, type I or type II diabetes
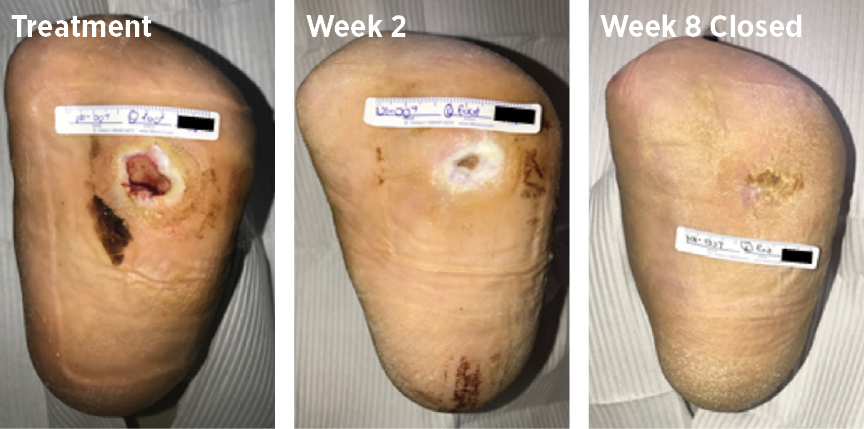
70-year-old male, with a DFU had been open for 90 weeks and had been previously treated with both 3 applications of EpiFix (MiMedx) and 1 treatment regimen of REGRANEX (Smith+Nephew); the ulcer healed in 8 weeks with MiroDerm.
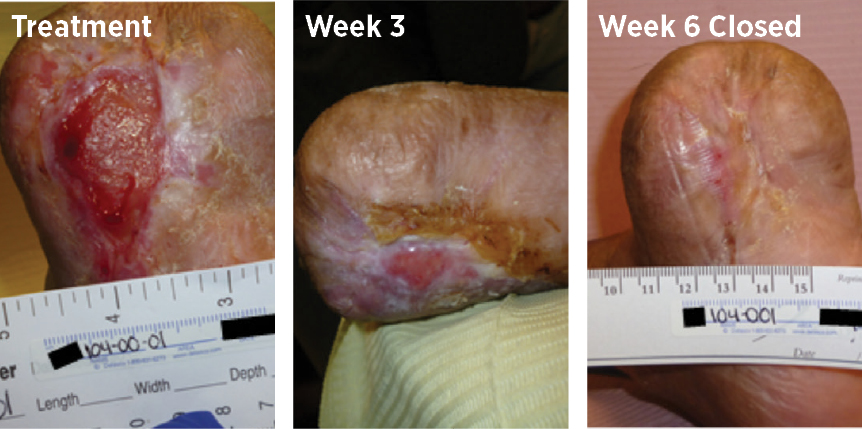
69-year old female, with a DFU that had been open for 34 weeks and had been previously treated with 2 applications of Omnigraft Dermal Regeneration Matrix (Integra LifeSciences) and 1 of CELLUTOME Epidermal Harvesting System (3M + KCI); the ulcer healed in 6 weeks with MiroDerm.
“The favorable results observed in this challenging patient population are promising with healing rates at or above the rates seen with typical DFUs and other advanced biologics.”
– Robert Fridman, DPM, Columbia University Medical Center
Contact us for more info on MiroDerm
1. Fridman, R; et al. Treatment of Hard-to-heal Diabetic Foot Ulcers With a Hepatic-derived Wound Matrix. Wounds. 2020.
2. Fridman, R; Engelhardt, J. A pilot study to evaluate the effects of perfusion-decellularized porcine hepatic-derived wound matrix on difficult-to-heal diabetic foot ulcers. Wounds.
2017. Vol. 29, No. 10. pp. 318-324.
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Reprise Biomedical and MiroDerm are trademarks or registered trademarks of Reprise Biomedical or its affiliates, in the U.S. and/or other countries. All other trademarks are property of their respective owners. ©2020 Reprise Biomedical. All Rights Reserved. SM-00113 Rev. E 09/20

