
Three-dimensional solution for deep and tunneling wounds
New HCPCS Level II Code A2025

Miro3D is designed for use in deep and tunneling wounds
- 2cm thick collagen sheet scaffold designed for deep, tunneling and irregular wounds
- Open and porous acellular graft provides protective environment for wound management
- Cut to desired size for full wall apposition and conformity to wound beds
Unlike thin grafts, Miro3D provides volume in deep wounds

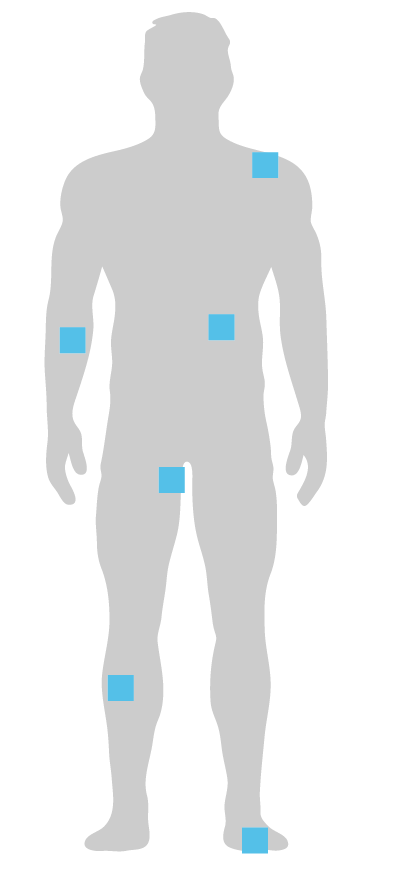
Broad range of clinical uses
- Stage III and IV pressure ulcers
- Necrotizing fasciitis
- Partial and full-thickness wounds
- Wound dehiscence
- Perirectal abscess
- Pilonidal sinus disease
- Diabetic foot ulcers
- Trauma wounds
- Other tunneled and undermined wounds
SAWC Fall Innovation Theater Sponsored by Reprise Biomedical:
Advancing 3D Wound Healing with Miro3D: Bridging Real-World Success with Clinical Evidence
Recorded live at the SAWC Fall 2025 Innovation Theater Friday, Sep. 4, 7:30-9:00am
Gain insights as leading wound care specialists, Dr. John Kirby, Dr. Robert Synder, Zwelithini Tunyiswa, BA and Taylor Stewart, CRNP present real-world cases and detail their use of Miro3D in a variety of complex wound treatment scenarios.
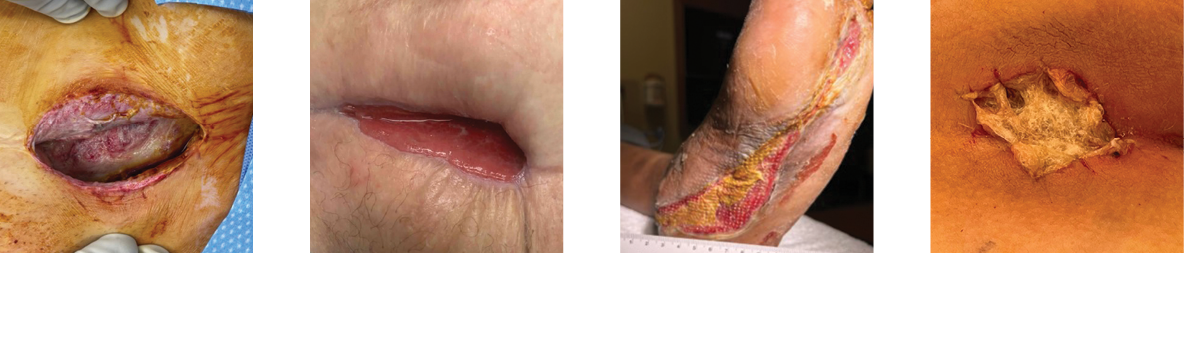
Clinical Case Studies and Posters*
Amputation Wound
Necrotizing Fasciitis
SAWC Fall 2023 Poster
Abdominal Surgical Dehiscence
Sebaceous Cyst
SAWC Spring 2024 Poster
Radiated Tissue Wound
Foot Abscess
SAWC Spring 2024 Poster
Foot Abscess
Pilonidal Sinus Abscess
SAWC Fall 2024 Poster
*The experience depicted in these case studies may not be representative of all potential clinical outcomes. Results may vary by patient. Further study is necessary to determine the benefit of Miro3D in a variety of wound applications.
Available in ten sizes (cm) that can be cut and shaped to fill and maintain direct contact with the wound bed

All sizes = 2cm thickness
Ordering Information
| ITEM ID | SIZE (cm) | TOTAL (cm3) | GTIN ID |
| 3000 | 2 x 2 x 2 | 8 | 00857072005316 |
| 3005 | 3 x 3 x 2 | 18 | 00857072005323 |
| 3006 | 5 x 2 x 2 | 20 | 00857072005620 |
| 3007 | 4 x 4 x 2 | 32 | 00857072005552 |
| 3009 | 10 x 2 x 2 | 40 | 00857072005637 |
| 3010 | 5 x 5 x 2 | 50 | 00857072005330 |
| 3011 | 6 x 5 x 2 | 60 | 00857072005644 |
| 3012 | 7 x 5 x 2 | 70 | 00857072005569 |
| 3013 | 8 x 5 x 2 | 80 | 00857072005651 |
| 3015 | 10 x 5 x 2 | 100 | 00857072005347 |
Packaged 1 per box.
GSA Contract #36F79721D0262
Miro3D wound matrix is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, partial thickness burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence). CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use. Download IFU here.
Available in a fiber version designed for irregular wound beds

Ordering Information
Miro3D Fibers wound matrix is intended for the management of wounds including: partial and full thickness wounds, pressure ulcers, venous ulcers, chronic vascular ulcers, diabetic ulcers, tunneled, undermined wounds, trauma wounds (abrasions, lacerations, partial thickness burns, and skin tears), draining wounds, surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence). CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use. Download IFU here.
Product Inquiries and Orders
Call us at: 763-284-6780 Fax us at: 952-856-5085 Product Inquiries at: customerservice@reprisebio.com
SM-00198 Rev. S 11/25


