
A porous and
compressible
wound matrix
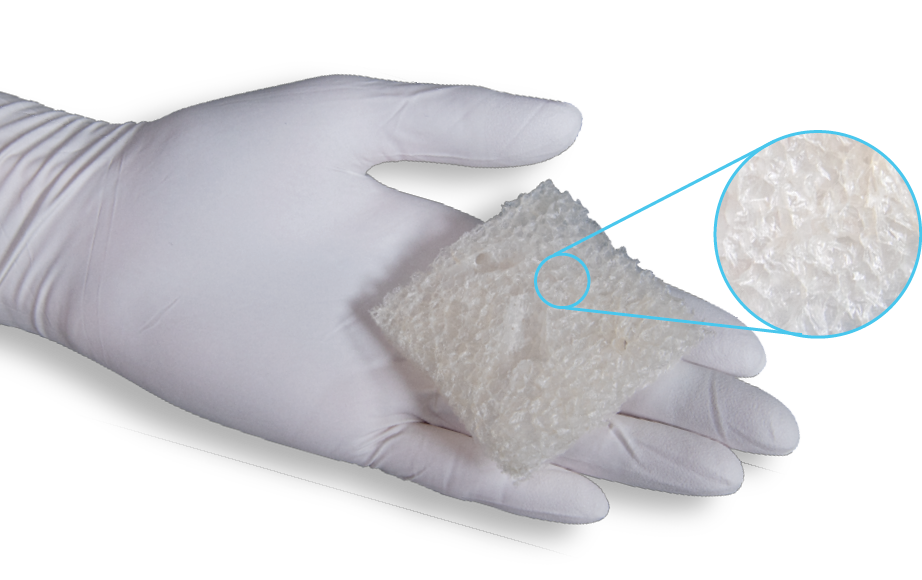
MiroDry is designed to conform to any wound bed
- Porcine collagen sheet scaffold
- Compressible and conformable to the irregular wound bed topography
- Innate porosity of the graft provides a protective environment for wound management
Broad range of clinical uses
- Partial and full-thickness wounds
- Diabetic foot ulcers
- Stage II, III and IV pressure ulcers
- Perirectal abscess
- Wound dehiscence
- Necrotizing fasciitis
- Pilonidal sinus disease
- Trauma wounds
- Other tunneled and undermined wounds

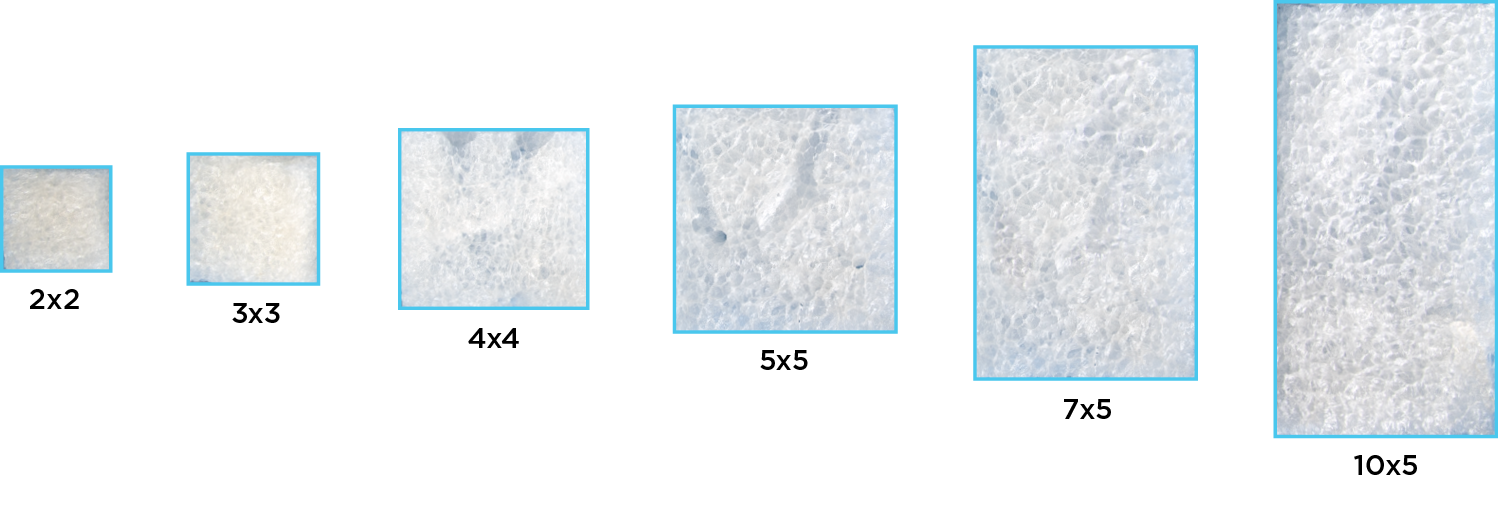
Available in six sizes (cm2)
Ordering Information
| ITEM ID | SIZE (cm)* | TOTAL (cm2) | GTIN ID |
| 6000 | 2 x 2 | 4 | 00857072005491 |
| 6005 | 3 x 3 | 9 | 00857072005507 |
| 6010 | 4 x 4 | 16 | 00857072005514 |
| 6015 | 5 x 5 | 25 | 00857072005521 |
| 6022 | 7 x 5 | 35 | 00857072005576 |
| 6025 | 10 x 5 | 50 | 00857072005545 |
Packaged 1 per box.
*Average thickness of 6.5mm
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Product Inquiries and Orders
Call us at: 763-284-6780 Fax us at: 952-856-5085 Product Inquiries at: customerservice@reprisebio.com
SM-00329 Rev. B 03/25
